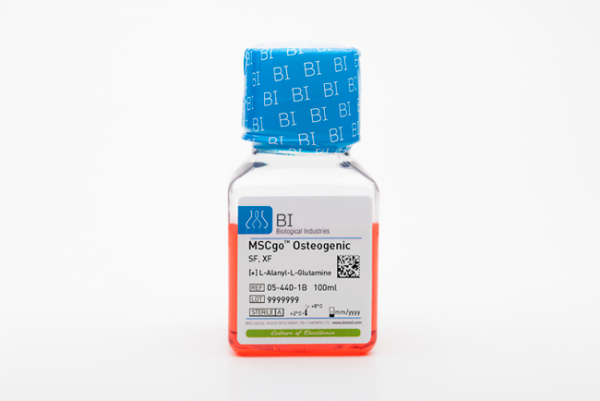
MSCgo Osteogenic Differentiation Medium
Complete, serum-free, xeno-free, ready-to-use medium
Optimized for directed differentiation of hMSCs to osteoblasts
- Description
- Specifications
- References
- Documentation
- Reviews (0)
Description
Product Overview
MSCgo™ Osteogenic Differentiation Medium is a serum-free (SF), xeno-free (XF), complete, and ready to use formulation developed for optimal differentiation of human mesenchymal stem cells (hMSC) to mature osteocytes. The MSCgo™ Osteogenic Differentiation Medium is validated to efficiently differeniate hMSC from a variety of sources, including bone marrow (BM-MSC), adipose tissue (AT-MSC), and umbilical cord tissue (UC-MSC).
The MSCgo™ Osteogenic Differentiation protocol is part of a complete system for multipotency evaluation of hMSCs. The MSCgo™ Osteogenic Differentiation Medium enables reliable osteogenesis of hMSCs. Differentiation to mature osteocytes is seen between 14 and 21 days.
Features
- Serum-free, xeno-free solution
- Complete differentiation medium
- Ready-to-use, simple protocol
- Reliable differentiation to mature osteocytes
- Contains stable L-alanyl-L-glutamine
- Does not contain antibiotics
Osteogenesis Results
Osteogenic differentiation of hMSC results in the formation of mineralized culture with calcified nodules and calcium secretion that can be detected with Alizarin Red S (ARS) staining. The ARS is used to stain calcium deposits formation which are an indication of mature osteocytes. The amount of calcified nodules formation and calcium secretion can be varying using different hMSC (e.g. source, age, and passage number).
Specifications
Required Materials for Osteogenic Assay
- MSCgo™ Osteogenic Differentiation Medium (BI Cat. No. 05-440-1) or MSCgo™ Rapid Osteogenic Differentiation Medium (BI Cat. No. 05-442-1)
- MSC NutriStem® XF Medium (BI Cat. No. 05-200-1)
- MSC Attachment Solution (BI Cat. No. 05-752-1)
- Alizarin Red S (ARS) – OPTIONAL
Legal
For human ex vivo tissue and cell culture processing applications. This reagent is not approved for human or animal use, or for application of in vitro diagnostic procedures.
Additional information
| Quantity | 100 mL |
|---|---|
| Brand | |
| Storage Conditions | 2 to 8°C |
| Shipping Conditions | Cold Pack |
| Quality Control | The MSCgo Osteogenic Differentiation Medium is validated for optimal differentiation of hMSC into osteocytes. Additional tests are: pH, osmolality, endotoxins and sterility tests |
- J. Leber et al., Microcarrier choice and bead-to-bead transfer for human mesenchymal stem cells in serum-containing and chemically defined media. Process Biochemistry, volume 59, Part B, August 2017, Pages 255-265
- L. Pu et al., Compared to the amniotic membrane, Wharton’s jelly may be a more suitable source of mesenchymal stem cells for cardiovascular tissue engineering and clinical regeneration. Stem Cell Research & Therapy 2017 8:72
- M. Meng et al., Umbilical cord mesenchymal stem cell transplantation in the treatment of multiple sclerosis. American Journal of Translational Research, 2018;10(1):212-223
Materials Safety Data Sheet
Manuals and Protocols
Be the first to review “MSCgo Osteogenic Differentiation Medium”
You must be logged in to post a review.






Reviews
There are no reviews yet.