Nutristem hPSC XF Medium
Defined, xeno-free, serum-free medium
Designed for optimal growth and expansion of human iPS and hES cells
| name | SKU | size |
|---|---|---|
| NutriStem® hPSC XF Medium, 500mL | 05-100-1A | 500 mL |
| NutriStem® hPSC XF Medium, 100mL | 05-100-1B | 100 mL |
| NutriStem® hPSC XF Medium (Growth Factor-Free) | 06-5100-01-1A | 500 mL |
- Description
- Specifications
- References
- Documentation
- Reviews (0)
Description
NutriStem hPSC Overview
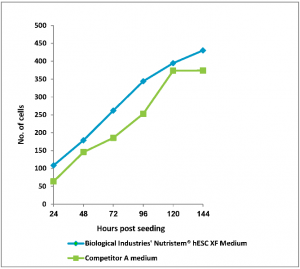
Figure: 1 Human Embryonic Stem Cells (H1, passage 6) were seeded in 96-well plates (Matrigel coated) in BI’s NutriStem hPSC XF and competitor’s media. Stem cell media were changed every 24 hours. Number of cells was determined using CyQuant™ cell proliferation assay kit.
NutriStem® hPSC XF Medium is a defined, xeno-free, serum-free cell culture medium designed to support the growth and expansion of human induced pluripotent stem (hiPS) and human embryonic stem (hES) cells. Nutristem hPSC is widely published and numerous protocols have been established for applications that incorporate the complete cell culture process from derivation to differentiation. NutriStem hPSC XF Medium allows for pluripotent stem cells to be cultured in a completely xeno-free system, without the need for high levels of basic FGF and other stimulatory growth factors and cytokines. The superior cell attachment and proliferation NutriStem hPSC XF Medium provides makes it an excellent medium choice to permit high-throughput screening applications. The reliable and consistent cellular morphology and growth of cells cultures in NutriStem hPSC XF Medium increases experimental reproducibility and has been shown in long-term growth of over 50 passages whilst preserving normal karyotypes.
NutriStem hPSC XF Features
- Defined, serum-free, and xeno-free
- Allows for flexibility to derived cells with a variety matrices
- Allows for weekend-free culture
- FDA Drug Master File (DMF) available, produced under cGMP
- Enables efficient expansion and growth of hES and hiPS cells in feeder-free culture systems
- Low-protein formulation that contains stable L-alanyl-L-glutamine and HSA
- Extensively tested and widely used on multiple hES and iPS cell lines, including H1, H9.2, I6, I3.2, and CL1
Sample Data
Cell morphology
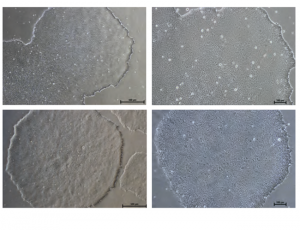
Figure: Normal Colony Morphology. H1 hES cells (top panel) and ACS-1014 hiPS cells (bottom panel) cultured in NutriStem® hPSC XF Medium on Matrigel-coated plates display colony morphologies typical of normal feeder-free hES and hiPS cell cultures, including a uniform colony of tightly compacted cells and distinct colony edges.
Immunostaining
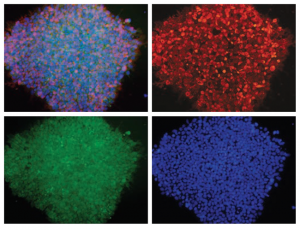
Figure: H1 cell morphology and immunofluorescence analysis of hESC markers red SSEA-4, green OCT4 and blue DAPI. H1 cells stained positive for the expression of pluripotency markers.
Embryoid body formation
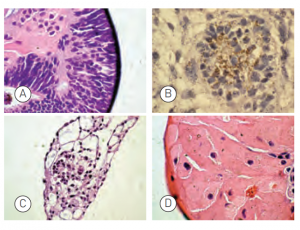
Figure: Embryoid bodies (EBs) were generated from H9.2 hES cells cultured for 16 passages in NutriStem® hPSC XF Medium on Matrigel matrix as an evaluation of pluripotency. The pluripotent H9.2 cells were suspended in serum-supplemented medium, where they spontaneously formed EBs containing cells of embryonic germ layers. The following cell types were identified by examination of the histological sections of 14-day-old EBs stained with H&E: (A) neural rosette (ectoderm), (B) neural rosette stained with Tubulin, (C) primitive blood vessels (mesoderm), and (D) megakaryocytes (mesoderm).
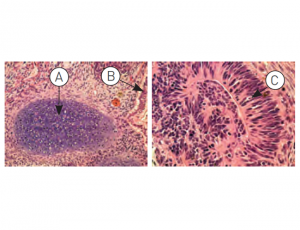
Figure: H9.2 hES cells were cultured for 11 passages in NutriStem® hPSC XF Medium using a human foreskin fibroblast (HFF) feeder layer. The hES cells were subsequently injected into the hind leg muscle of SCID-beige mice for in vitro evaluation of pluripotency. The following tissues from all three germ layers were identified in H&E-stained histological sections of the teratoma 12 weeks post-injection: (A) cartilage (mesoderm), (B) epithelium (endoderm), and (C) neural rosette (ectoderm).
Instructions for Use
- Upon thawing, the medium may be stored at 2 – 8ºC for 10 to 14 days
- Media should be aliquotted into smaller working volumes to avoid repeated freeze/thaw cycles
- Avoid exposure to light
Note: A common feeder-free basement membrane matrix is Matrigel, which is not xeno-free. Effective xeno-free alternatives to Matrigel is recombinant laminin, such as LaminStem(R) 521 (BI Cat. No. 05-753-1F) which has been validated to successfully culture human ES and iPS cells using NutriStem® hPSC XF medium.
Legal : NutriStem® hPSC XF is registered as an In-vitro diagnostic (IVD) medical device. NutriStem® is a registered trademark of Biological Industries.
A Drug Master File (DMF) for NutriStem® hPSC XF is available.
Additional information
| Brand | |
|---|---|
| Form | Liquid |
| Storage Conditions | Store at -20ºC |
| Shipping Conditions | Dry Ice |
| Quality Control | NutriStem® hPSC is routinely tested for optimal maintenance and expansion of undifferentiated hESCs. Additional standard evaluations are pH, osmolality, endotoxins and sterility tests. |
Growing Methods of hESC and iPSC (Derivation, Expansion, Scaling up, and Suspensions)
- O.M. Russell et al. Preferential amplification of a human mitochondrial DNA deletion in vitro and in vivo. Scientific Reports, volume 8, Article number: 1799 (2018)
- J. Rosati et al. Production and characterization of human induced pluripotent stem cells (iPSCs) from Joubert Syndrome: CSSi001-A (2850). Stem Cell Research, Volume 27, March 2018, Pages 74–77
- Maroof M Adil, David V Schaffer. Expansion of human pluripotent stem cells. Current Opinion in Chemical Engineering 2017, 15:24–35
- Tateno, H. et al. Development of a practical sandwich assay to detect human pluripotent stem cells using cell culture media Regenerative Therapy, Volume 6, June 2017, Pages 1–8
- Baker, D. et al. Detecting Genetic Mosaicism in Cultures of Human Pluripotent Stem Cells Stem Cell Reports, 2016
- Vega-Crespo, A., et al. Investigating the functionality of an OCT4-short response element in human induced pluripotent stem cells. Molecular Therapy — Methods & Clinical Development 3, Article number: 16050 (2016)
- Y.Y. Lipsitz, P.W. Zandstra, Human pluripotent stem cell process parameter optimization in a small scale suspensionbioreactor. BMC Proceedings, 9(Suppl 9), O10, 2015
- S. Gregory et al. Autophagic response to cell culture stress in pluripotent stem cells. Biochemical and Biophysical Research Communications, doi:10.1016/j.bbrc.2015.09.080, 2015
- N. Desai, P Rambhia and A. Gishto, Human embryonic stem cell cultivation: historical perspective and evolutionof xeno-free culture systems. Reproductive Biology and Endocrinology 13.1 (2015): 9.
- T. Yokobori et al., Intestinal epithelial culture under an air-liquid interface: a tool for studying human and mouse esophagi. Diseases of the Esophagus, doi: 10.1111/dote.12346. 2015
- L. Healy, L Ruban, Derivation of Induced Pluripotent Stem Cells, Atlas of Human Pluripotent Stem Cells in Culture, pp 149-165. Springer US 2015
- W. Siqin et al. Spider silk for xeno-free long-term self-renewal and differentiation of human pluripotent stem cells. Biomaterials 35.30 (2014): 8496-8502.
- G. Finesilver, M. Kahana, E. Mitrani. Kidney-Specific Micro-Scaffolds and Kidney Derived Serum FreeConditioned Media support in vitro Expansion, Differentiation, and Organization of Human Embryonic Stem Cells. Tissue Engineering Part C: Methods. -Not available-, ahead of print. doi:10.1089/ten.TEC.2013.0574.
- M. Amit, J. Itskovitz-Eldor. Atlas of Human Pluripotent Stem Cells: Derivation and Culturing. Stem Cell Biology and Regenerative Medicine, 2012
- R. Bergström, Xeno-free culture of human pluripotent stem cells, Methods Mol Biol. 2011;767:125-36
- J.Collins et al,. Highly Efficient Reprogramming to Pluripotency and Directed Differentiation of Human Cells withSynthetic Modified mRNA. Cell Stem Cell 7 (5): 618-630 (2010).
- K. Jacobs et al. Higher-Density Culture in Human Embryonic Stem Cells Results in DNA Damage and GenomeInstability. Stem Cell Reports: 6(3), pp 330–341, 2016
Differentiation of Pluripotent Stem Cells
- K.M. Gray et al. Self-oligomerization regulates stability of Survival Motor Neuron (SMN) protein isoforms by sequestering an SCFSlmb degron. Molecular Biology of the Cell, 2017 mbc.E17-11-0627
- D.C. Wilkinson et. al. Development of a Three-Dimensional Bioengineering Technology to Generate Lung Tissue for Personalized Disease Modeling. Stem cells translational medicine, 6(2), 2017
- E. Welby et al. Isolation and Comparative Transcriptome Analysis of Human Fetal and iPSC-Derived Cone Photoreceptor Cells. Stem Cell Reports (2017), https://doi.org/10.1016/j.stemcr.2017.10.018
- R. De-Santis et. al. FUS Mutant Human Motoneurons Display Altered Transcriptome and microRNA Pathways with Implications for ALS Pathogenesis. Stem Cell Reports (2017), https://doi.org/10.1016/j.stemcr.2017.09.004
- R.A. Hazim et al. Differentiation of RPE cells from integration-free iPS cells and their cell biological characterization. Stem Cell Research & Therapy 2017
- S. Petrus-Reurer et al. Integration of Subretinal Suspension Transplants of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells in a Large-Eyed Model of Geographic Atrophy. Retinal Cell Biology, February 2017
- X. Yuan et al. A hypomorphic PIGA gene mutation causes severe defects in neuron development and susceptibility to complement-mediated toxicity in a human iPSC model, PLOS ONE, 2017
- Lenzi, J., et al. Differentiation of control and ALS mutant human iPSCs into functional skeletal muscle cells, a tool for the study of neuromuscolar diseases. Stem Cell Research: Volume 17, Issue 1, Pages 140–147, 2016.
- K. Alessandri et. al. A 3D printed microfluidic device for production of functionalized hydrogel microcapsules forculture and differentiation of human Neuronal Stem Cells (hNSC). Lab on a Chip: 16(9), 2016
- D. Voulgaris, Evaluation of Small Molecules for Neuroectoderm differentiation & patterning using Factorial Experimental Design. Master Thesis in Applied Physics, Department of Physics, Division of Biological Physics, Chalmers University of Technology, Göteborg, Sweden 2016
- P. Bergström et al. Amyloid precursor protein expression and processing are differentially regulated during cortical neuron differentiation, Scientific Reports, 2016
- Tieng, V. ae al. Elimination of proliferating cells from CNS grafts using a Ki67 promoter-driven thymidine kinase, Molecular Therapy — Methods & Clinical Development 6, Article number: 16069, 2016
- Brykczynska, U, et al. CGG Repeat-Induced FMR1 Silencing Depends on the Expansion Size in Human iPSCs and Neurons Carrying Unmethylated Full Mutations Stem Cell Reports, 2016
- Sellgren ,C.M. et al. Patient-specific models of microglia-mediated engulfment of synapses and neural progenitors Molecular Psychiatry, 2016
- Cosset, E. et al. Human tissue engineering allows the identification of active miRNA regulators of glioblastoma aggressiveness Biomaterials, 2016
- M. Di Salvio et al. Pur-alpha functionally interacts with FUS carrying ALS-associated mutations. Cell Death & Disease, 2015
- A. Reyes, et al. Xeno-Free and Defined Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells Functionally Integrate in a Large-Eyed Preclinical Model Plaza. Stem Cell Reports: Volume 6, Issue 1, p9–17, 2015
- Tieng, V. et al.Engineering of Midbrain Organoids Containing Long-Lived Dopaminergic Neurons. Stem Cells and Development. February 2014, 23(13): 1535-1547.
- A. J. Schwab, A.D. Ebert, Sensory Neurons Do Not Induce Motor Neuron Loss in a Human Stem Cell Model of SpinalMuscular Atrophy. PLoS One. 2014; 9(7): e103112
- H.X. Nguyen et al., Induction of early neural precursors and derivation of tripotent neural stem cells from humanpluripotent stem cells under xeno-free conditions. Journal of Comparative Neurology: Volume 522, Issue 12, pp 2767–2783, 2014
- A. Kurtz, A. Bosio, and S. Knoebel. Highly efficient differentiation of hPSC into hepatocyte-like cellsby selection of CXCR4 (CD184) definitive endoderm (DE) cells
Cardiomyocyte differentiation
- R. Ophir et al. Inflammation And Contractility Are Altered By Obstructive Sleep Apnea Children's Serum, In Human Embryonic Stem Cell Derived Cardiomyocytes. American Journal of Respiratory and Critical Care Medicine 2017
- J. Kristensson, Optimization of Growth Conditions for Expansion of Cardiac Stem Cells Resident in the Adult Human Heart. Master’s thesis in Biotechnology, Department of Physics, Division of Biological Physics, Chalmers University of Technology, Gothenburg, Sweden 2016
- S. Rajasingh et al. Generation of Functional Cardiomyocytes fro Efficiently Generated Human iPSCs and a Novel Method of Measuring Contractility. PloS one 10.8, 2015: e0134093
- L. Jacquet et al. Three Huntington’s Disease Specific Mutation-Carrying Human Embryonic Stem Cell Lines Have Stable Number of CAG Repeats upon In Vitro Differentiation into Cardiomyocytes. PloS one 10.5, 2015
- V. Bellamy et al., Long-term functional benefits of human embryonic stem cell-derived cardiac progenitors embedded into a fibrin scaffold, The Journal of Heart and Lung Transplantation, 2014, in press
- E. Di Pasquale et al. Generation of human cardiomyocytes: a differentiation protocol for feeder-free human induced pluripotent stem cells. JoVE (Journal of Visualized Experiments) 76 (2013): e50429-e50429
- P.W. Burridge and E.T Zambidis. Highly efficient directed differentiation of human induced pluripotent stem cells into cardiomyocytes. Pluripotent Stem Cells: Methods and Protocols. Methods in Molecular Biology, volume 997, pp 149-161, Humana Press, 2013.
Gene Editing
- Sweeney, CL et al.Targeted Repair of CYBB in X-CGD iPSCs Requires Retention of Intronic Sequences for Expression and Functional Correction,Molecular Therapy, 2017
- J. Lenzi et al., ALS mutant FUS proteins are recruited into stress granules in induced Pluripotent Stem Cells (iPSCs) derived motoneurons. Disease Models & Mechanisms: 8, 755-766, 2015
- T. Cerbini et al., Transfection, Selection, and Colony-picking of Human Induced Pluripotent Stem Cells TALEN-targetedwith a GFP Gene into the AAVS1 Safe Harbor, JoVE (Journal of Visualized Experiments), 2015
- Y Qin, et al. Laminins and cancer stem cells: partners in crime? Seminars in Cancer Biology, 2016
- S. Wu et al. Efficient passage of human pluripotent stem cells on spider silk matrices under xeno-free conditions. Cellular and Molecular Life Sciences: 73(7):1479-88, 2015
- O. Simonson. Use of Genes and Cells in Regenerative Medicine. Karolinska Institutet, 2015
- Nacalai USA Inc. Vitronectin-398™ (Xeno-free). Nacalai USA website
- S. Rodin et al., Monolayer culturing and cloning of human pluripotent stem cells on laminin-521–based matrices under xeno-free and chemically defined conditions. Nature Protocols 9, 2354–2368 (2014) doi:10.1038/ nprot.2014.159
- Rodin S, et al. Clonal culturing of human embryonic stem cells on laminin-521/E-cadherin matrix in defined and xeno-free environment. Nat Commun. 5:3195. doi: 10.1038/ncomms4195, 2014
- StemAdhere™ Defined Matrix for hPSC. Primorigen Biosciences website.
- I. lenz et al., Automated 3D Culture to Undifferentiated hESC. (Scientific Poster)
- J.L. Weber et al,. The Corning® Synthemax™ Surface: A Synthetic, Xeno-Free Surface for Long-Term Self-Renewal of Human Embryonic Stem Cells in Defined Media. presented in 2010 world stem cell summit
Induction of Pluripotency of hESC and iPSC
- X. Gao et. al. Comparative transcriptomic analysis of endothelial progenitor cells derived fro umbilical cord blood and adult peripheral blood: Implications for the generation of induced pluripotent stem cells. Stem Cell Research, 2017
- M.V. Krivega et al. Cyclin E1 plays a key role in balancing between totipotency and differentiation in human embryonic cells. Mol. Hum. Reprod, 2015
- S. Herz, Optimization of RNA-based transgene expression by targeting Protein Kinase R. Dissertation for the degree “Doctor rerum naturalium”, 2015
- S. Eminli-Meissner et al. A novel four transfection protocol for deriving iPS cell lines fro human blood- derivedendothelial progenitor cells (EPCs) and adult human dermal fibroblasts using a cocktail of non-modified reprogrammingand immune evasion mRNAs. Sientific Poster, REPROCELL, 2015
- M. Brouwer et al. Choices for Induction of Pluripotency: Recent Developments in Human Induced Pluripotent StemCell Reprogramming Strategies. Stem Cell Reviews and Reports: Volume 12, Issue 1, pp 54–72, 2015
- R. S. Song et al. Generation, Expansion, and Differentiation of Human Induced Pluripotent Stem Cells (hiPSCs) Derived fro the Umbilical Cords of Newborns. Current protocols in stem cell biology (2013): UNIT 1C.16.
- Warren, L., et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 7: 618-630, 2010
- S. Sugii et al., Human and mouse adipose-derived cells support feeder-independent induction of pluripotent stem cells. PNAS February 23, 2010 vol. 107 no. 8 3558-3563
Proteins and Antibodies Expression and Isolation
- D.R. Riordon and K.R. Boheler, Immunophenotyping of Live Human Pluripotent Stem Cells by Flow Cytometry. In: Boheler K., Gundry R. (eds) The Surfaceome. Methods in Molecular Biology, vol 1722. Humana Press, New York, NY, 2018
- N.Y. Thakar et al., TRAF2 recruitment via T61 in CD30 drives NFkB activation and enhances hESC survival andproliferation, Molecular Biology of the Cell: 26(5):993-1006 2015
- Abcam, Immunocytochemistry / Immunofluorescence abreview for Anti-Oct4 antibody - ChIP Grade. Abcam website
Clinical Applications- Derivation and Expansion of hESC and IPSC
- L. de Oñate et al. 2015. Research on Skeletal Muscle Diseases Using Pluripotent Stem Cells. DOI: 10.5772/60902
- P. Menasché et al., Towards a Clinical Use of Human Embryonic Stem Cell-Derived Cardiac Progenitors:A Translational Experience. European Heart Journal: Volume 36, Issue 12, pp 743-50, 2015
- P. Menasché et al. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. European heart journal (2015): ehv189.
- T. Seki, K. Fukuda, Methods of induced pluripotent stem cells for clinical application, World Journal of Stem Cells: Volume 7, Issue 1, pp 116–125, 2015
- Y. Luo et al.,Stable Enhanced Green Fluorescent Protein Expression After Differentiation and Transplantation of Reporter Human Induced Pluripotent Stem Cells Generated by AAVS1 Transcription Activator-Like Effector Nucleases, STEM CELLS Translational Medicine: Volume 3, Issue 7, pp 821-35, 2014
- J. Durruthy-Durruthy et al. Rapid and Efficient Conversion of Integration-Free Human Induced Pluripotent Stem Cells to GMP-Grade Culture Conditions. PlOS one: http://dx.doi.org/10.1371/journal.pone.0094231, 2014
- H. Tateno et al. A medium hyperglycosylated podocalyxin enables noninvasive and quantitative detection of tumorigenic human pluripotent stem cells. Scientific Reports 4, Article number: 4069, 2014
- S. Abbasalizadeh, H. Baharvand. Technologies progress and challenges towards cGMP manufacturing of human pluripotent stem cells based therapeutic products for allogeneic and autologous cell therapies. Biotechnology Advances: Volume 31, Issue 8, pp 1600-23, 2013.
- J. P. Awe et al. Generation and characterization of transgene-free human induced pluripotent stem cells and conversion to putative clinical-grade status. Stem Cell Research & Therapy, 2013, 4:87
- O. Hovatta. Infectious problems associated with transplantation of cells differentiated fro pluripotent stem cells. Seminars in Immunopathology: Volume 33, Issue 6, pp 627-30, April 2011
- S. Ström. Optimisation of human embryonic stem cell derivation and culture – towards clinical quality. Karolinska Institutet, Stockholm, Sweden, 2010
Drug Screening
- Z. Ye et al., Differential sensitivity to JAK inhibitory drugs by isogenic human erythroblasts and hematopoieticprogenitors
Animal Models
- W. Zhang, et al. Distinct MicroRNA Expression Signatures of Porcine Induced Pluripotent Stem Cells under Mouse and Human ESC Culture Conditions. PLOS ONE, 2016
Patents
- J. Mata-fink et al. Methods and Compositions For Immunomodulation. US Patent Application 20170020926, 2017
- Bailly, J., et al. Method for differentiation of pluripotent stem cells into multi-competent renal precursors. US Patent 20,160,145,578, 2016
- K. Tryggvason et al. Differentiation of pluripotent stem cells and cardiac progenitor cells into striated cardiomyocyte fibers using laminins LN-511, LN-521 and LN-221. US Patent Application 20160122717, 2016
- D. Keefe et al. A Method for a Single Cell Analysis of Telomere Length. US Patent Application 20160032360, 2016
- Dietrich M. EGLI, Methods for making and using modified oocytes . US Patent Application 20140308257, 2016
- M. Mikkola et. al. A Method for Generating Induced Pluripotent Stem Cells. US Patent Application 20160068818, 2016
- H Tateno et al. Undifferentiated Cell Detection Method and Complex Carbohydrate Detection Method. US Patent Application 20150204870, 2015
- O. Hovatta, K. Tryggvason, Methods of Producing RPE Cells. US Patent Application 20150299653, 2015
- A. De Fougerolles, S.M. Elbashir, Delivery and Formulation of Engineered Nucleic Acids, US Patent Application 20150017211, 2015
- Sahin, U., et al. Diagnosis and therapy of cancer involving cancer stem cells. US Patent Application 20150314018, 2015
- U. Sahin et al. Method for Cellular RNA Expression. US Patent Application 20150314018, 2015
- M.F Yanik, M. Angel. Methods for Transfecting Cells With Nucleic Acids. US Patent Application 20140073053, 2014
- C.E Buensuceso et al., Induced Pluripotent Stem Cells Prepared fro Human Kidney-Derived Cells. US Patent Application 20140073049, 2014
- C. Buensuceso et al., Induced Pluripotent Stem Cells fro Human Umbilical Cord Tissue-Derived Cells. US Patent Application 20130157365, 2013
- A. De Fougerolles, S.M. Elbashir, J.P. Schrum. Modified Polynucleotided for the Production of Factor IX. US Patent Application 20130245103, 2013
- M. Amit, J. Itskovitz-eldor. Novel Methods and Culture Media for Culturing Pluripotent Stem Cells. US Patent Application 20130236961, 2013
- E. Zambidis, P Burridge. Compositions and Methods of Generating a Differentiated Mesodermal Cell. US Patent Application 20130136721, 2013.
Material Safety Data Sheet
Manuals and Protocols
- Weekend-free culture of human pluripotent stem cells on LN-521 (BioLamina)
- NutriStem® hPSC XF Medium Instructions for Use
- NutriStem® hPSC XF Medium Technical Manual
Webinars & Videos
- On-Demand Webinar: Human Pluripotent Stem Cells (hPSC) – An Overview of Morphology and Culture Methods (BI-USA)
- On-Demand Webinar: Reprogramming and iPSC culture considerations for translational research (RegMedNet)
- Protocol video: Passaging Human Embryonic Stem Cells and Induced Pluripotent Stem Cells in NutriStem® XF/FF Culture Medium (Stemgent)
Product Literature
- NutriStem® hPSC XF Medium Product Sheet
- Case Study: Xeno-Free hiPS Cell Culture using NutriStem® hPSC XF Medium on a Human Vitronectin Substrate without the need for ROCK Inhibitors
- Application Note: Corning® PureCoat™ rLaminin-521 Cultureware: A Ready-to-use, Animal-free Vessel Platform for the Culture and Single Cell Passaging of Human Pluripotent Stem Cells (Corning)
- Application Note: NutriStem® hPSC XF Medium Supports Longterm Culture of Human Pluripotent Stem Cells (Corning)
- Application Note: Single Cell Passaging with NutriStem® hPSC XF on Laminin-521 without the Need for ROCK Inhibitor
- Technical Resources: Passaging Methods for hPSCs under Feeder-Free Conditions
Frequently Asked Questions
Be the first to review “Nutristem hPSC XF Medium”
You must be logged in to post a review.









Reviews
There are no reviews yet.